Researchers from the University of Chicago have discovered the rare change in business as a potential educational path, and have identified excellent hope for the deaf.
A team of scientists at the University of Chicago, in collaboration with the University of Miami, has gained research expertise in a way that is not often heard. The team has also succeeded in identifying two new treatments that will once again close the door to hope for the inherited disease.
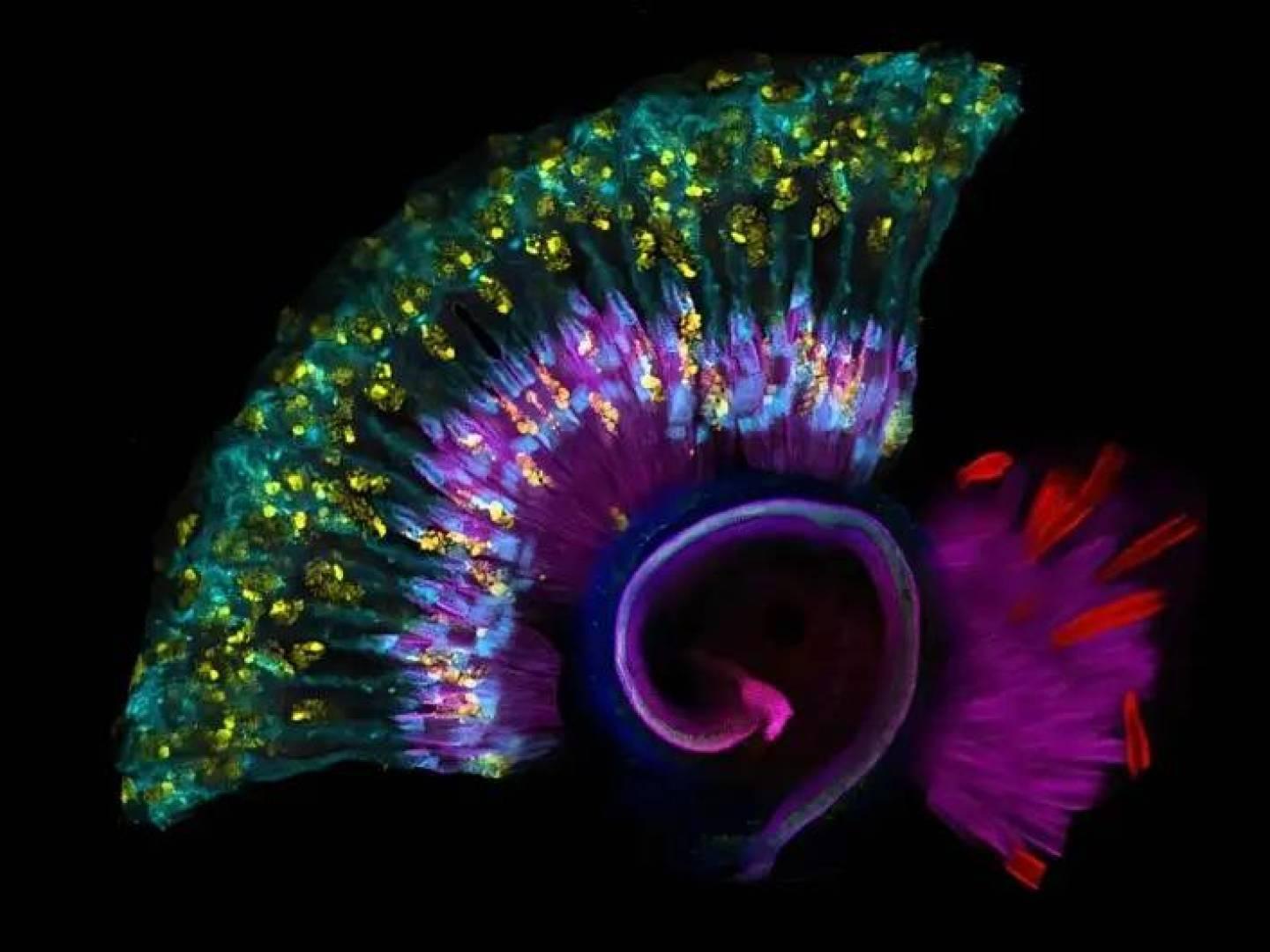
The results of the study were published in the Journal of Clinical Investigation and revealed that the CPD gene, known for its protein-modifying function, also affects the inner ear, causing disturbances in the sensory cells responsible for hearing.
Dr.Rong Grace Zhai, Professor of Neuroscience at the University of Chicago and Leader of the research team, said:
"This is an exciting discovery because we have not only discovered a new genetic mutation associated with deafness, but we have also identified a therapeutic target that could alleviate this condition."
CPD GENE and COURSE PLAN
Researchers began studying the CPD gene after noticing a distinct group of mutations in three unrelated Turkish families with a type of hearing loss known as sensorineural deafness (SNHL), a genetic condition that often appears in early childhood and leads to permanent hearing loss.
Although the investment in pochlear can improve some of the patients, the lack of effective treatment in others causes an increase in the same seed and shows signs of toxic loss.
Human cells and arginine production
Researchers conducted experiments on mice to understand the CPD gene's ability to hear.
This gene normally produces an enzyme that makes the amino acid arginine, which in turn promotes the production of nitric oxide, an important neurotransmitter that helps transmit signals in the nervous system.
But when mice are exposed to mutations in this gene, this vital process is disrupted, leading to oxidative stress and the death of sensory cells in the ear, especially in the fine hairs responsible for receiving sound waves.
"The CPD gene maintains arginine levels in auditory hair cells to enable rapid nerve signaling through nitric oxide production."
“Therefore, these cells become more fragile when this gene is absent compared to other neurons,” explains Zhai.
The Fly Woh model adds therapeutic benefits
Scientists have also experimentally demonstrated the effects of railroad tracks, and those affected have shown movement and balance disturbances similar to those caused by damage to human ears.
The team then tested two possible treatments:
1. Supplement arginine to compensate for deficiency due to mutations.
2. Sildenafil (also known as Viagra) stimulates neural pathways affected by the loss of nitric oxide.
The results showed that both treatments improved cell survival and reduced hearing loss in fruit flies.
"What makes this discovery so powerful is that not only have we understood the mechanism of deafness at the cellular and molecular level, but we have also found a promising therapeutic avenue with drugs already approved by the US Food and Drug Administration," Zhai said.
New perspectives in understanding age-related hearing loss
The scientific team plans to continue their work to study the role of nitric oxide in the reproductive system of the inner ear and to study CPD crimes around the world.
"This gene may be a risk factor for age-related hearing loss or other types of sensory neuropathy, making it a future scientific priority," Zhai concludes.